
In the pharmaceutical industry, data integrity is a central element in ensuring drug quality, patient safety, and compliant operations. From R & D to production, from clinical trials to circulation traceability, the data of each link need to be true, accurate and traceable. As the "first line of defense" of enterprise information system, password management is directly related to the access rights and security of data. How to meet regulatory requirements such as GMP, GxP and FDA 21 CFR Part 11 with a scientific password policy?
一、 Regulatory requirements: Password management is not a "multiple choice question"
1. FDA 21 CFR Part 11:
O § 11.300: Electronic signatures must be authenticated by a unique identifier (e.g. username + password), and their security and non-repudiation must be ensured.
O § 11.10 (d): The system is required to protect against unauthorized operations through access controls such as password policies.
2. EU GMP Annex 11:
O Annex 11, Chapter 6: Computerized systems need to ensure data security and traceability through rights management (e.g. password control).
O Appendix 11, Chapter 9: All user actions need to be logged and password policies should be protected against unauthorized access and tampering.
3. WHO GMP:
Chapter 5: Critical systems need to protect data integrity through user authentication, such as passwords.
O Chapter 17: Password policies should prevent unauthorized access and support audit trails.
4. Good Manufacturing Practice (GMP):
O Article 163: Computerized systems shall be equipped with operating authority management to prevent data tampering or deletion.
O Appendix "Computerized System": User accounts shall be authenticated by password, and the password policy shall comply with the data security requirements.
5. Drug Record and Data Management Requirements (Trial) (NMPA):
O Article 14: The authenticity and integrity of electronic data shall be ensured through access control (e.g. password).
O Article 23: The system shall record the user login and operation log, and the password policy shall support the audit trail.
Password management is not only a technical problem, but also the "lifeline" of compliance of pharmaceutical enterprises. From FDA 21 CFR Part 11 to Chinese GMP, regulations emphasize that password policies need to be closely aligned with data integrity objectives.
二、Risk Analysis: Start with Password Vulnerability
If pharmaceutical companies adopt a "loose" password policy, the following risks may arise:
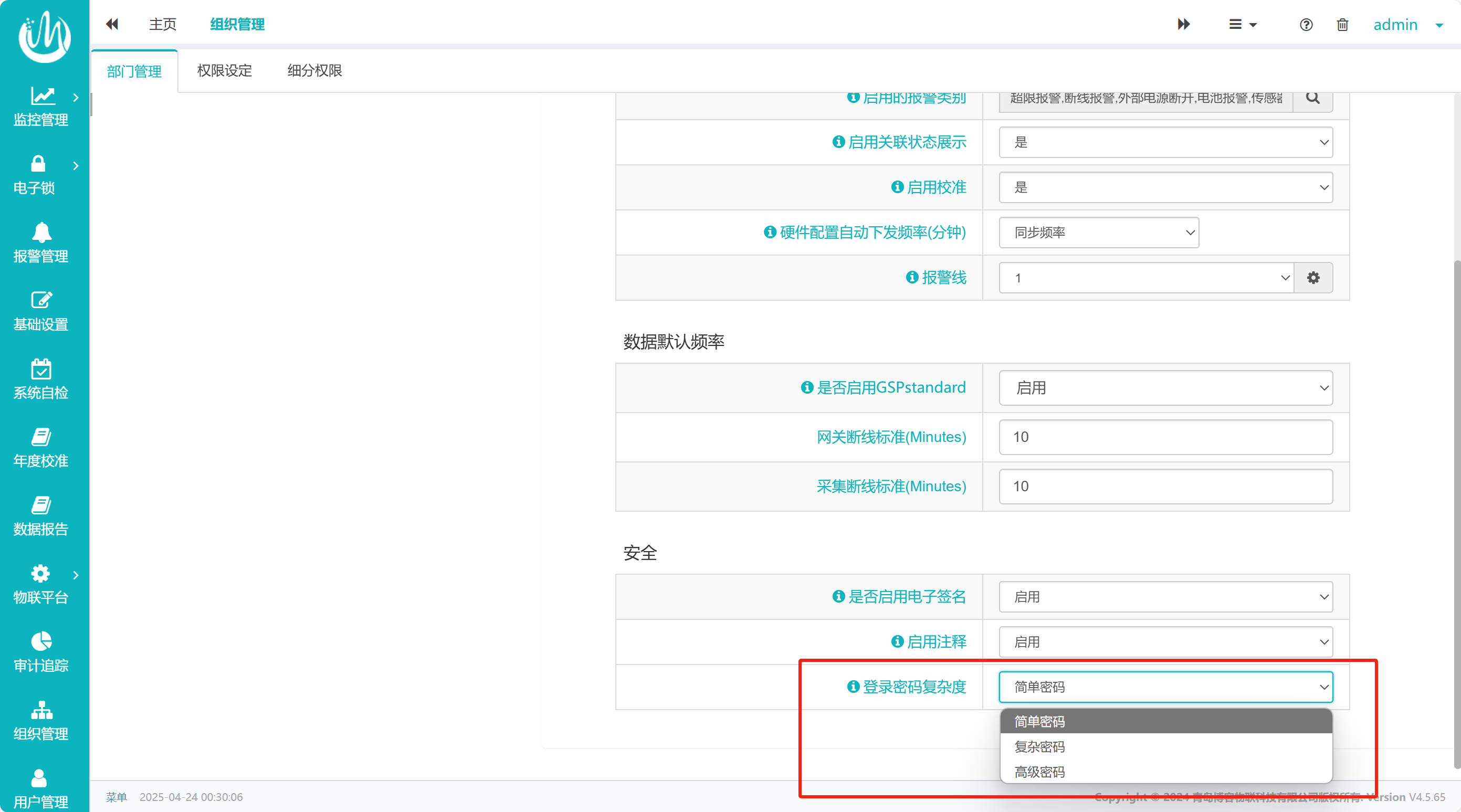
1. Account sharing and fraudulent use: The configuration of allowing "multi-user login" (such as simple policy) can easily lead to untraceable operation, which violates the audit principle of "one person, one account".
2. Risk of brute force cracking: 10 wrong attempts (simple strategy) are allowed, which is much higher than 5 limit of complex strategy. Attackers may gain access through multiple trial and error.
3. Hidden danger of static password: no SMS authentication or two-factor authentication (such as simple strategy), only relying on a single password, it is difficult to resist external attacks.
Enterprises need to build a strong line of defense for data security through scientific password configuration (such as complexity, validity, dynamic verification) and strict management system to avoid compliance risks caused by password vulnerabilities.
三、Compliance password management: password policy settings
Based on the above regulations and risks, the computer systems of pharmaceutical enterprises should adopt strict password policy settings. Ciplelink continuous monitoring system can be roughly divided into three strategies: simple, complex and advanced. The specific content is suggested from several aspects:
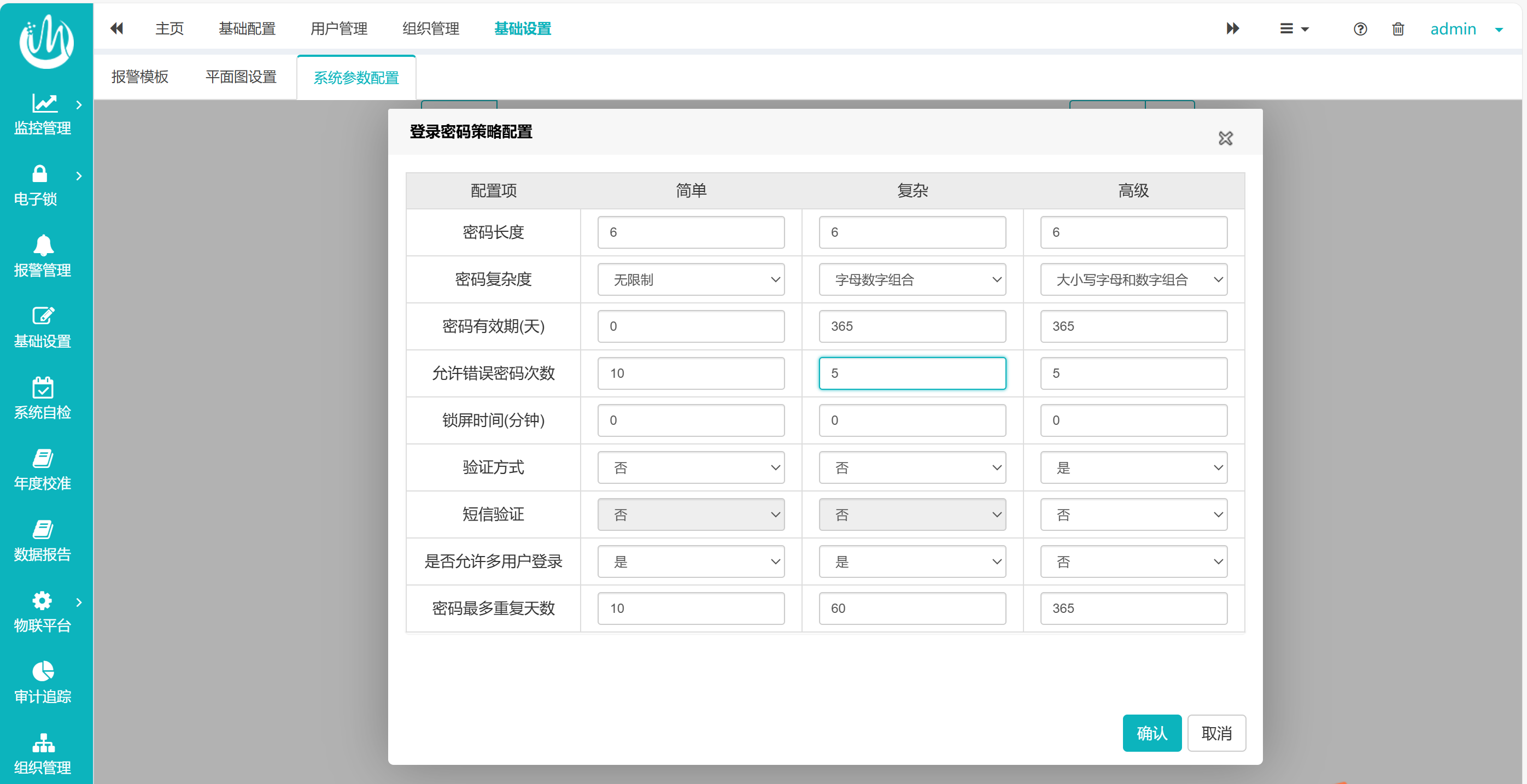
Complexity and length: The combination of upper and lower case letters and numbers is mandatory, and the length is recommended to be extended to multiple digits to reduce the risk of password cracking.
Allowed error times: The account will be automatically locked after the user enters the wrong password times beyond the specified range, so as to improve the security of the account.
Validity period and repeat limit: set the password validity period and password repeat period, force users to update regularly, and reduce long-term exposure risks.
Dynamic authentication: Enable SMS authentication or biometrics for two-factor authentication, meeting FDA requirements for "trustworthy systems.".
Permission quarantine: Multiple user logins are prohibited to ensure that the operation can be traced back to the specific responsible person.
Automatic screen locking and aging: It is recommended to shorten the screen locking time to avoid unauthorized access when the device is idle.
Take the example of the password policy applied to the computerized system in the pharmaceutical industry to meet the regulatory requirements. This system can configure different levels of password policies according to the importance of the account level (see Figure 1), and the specific policy content can be customized according to the user's needs (see Figure 2):
Under the tide of digitalization, the password management of computer system has been upgraded from "technical details" to the strategic link of compliance operation of pharmaceutical enterprises. Only through scientific strategy allocation and continuous monitoring and optimization, can we build the cornerstone of data integrity and escort the whole life cycle of drugs. Starting today, review your password policy — is it sufficient to protect against risk? Can it stand the test of laws and regulations? The answer, perhaps, is hidden in the details of every password entry, which Biorong Ciplelink continuously monitors: details determine success or failure.
Copyright©2019Qingdao Biorong IoT Technology Co., Ltd All right reserved 鲁ICP备20030433号-1 Website Map